TECNIS Eyhance™ IOL
Also available in Toric II.
Also available in Toric II.
TECNIS Eyhance™ offers Distance PLUS* without compromise** 1-3
Key Features
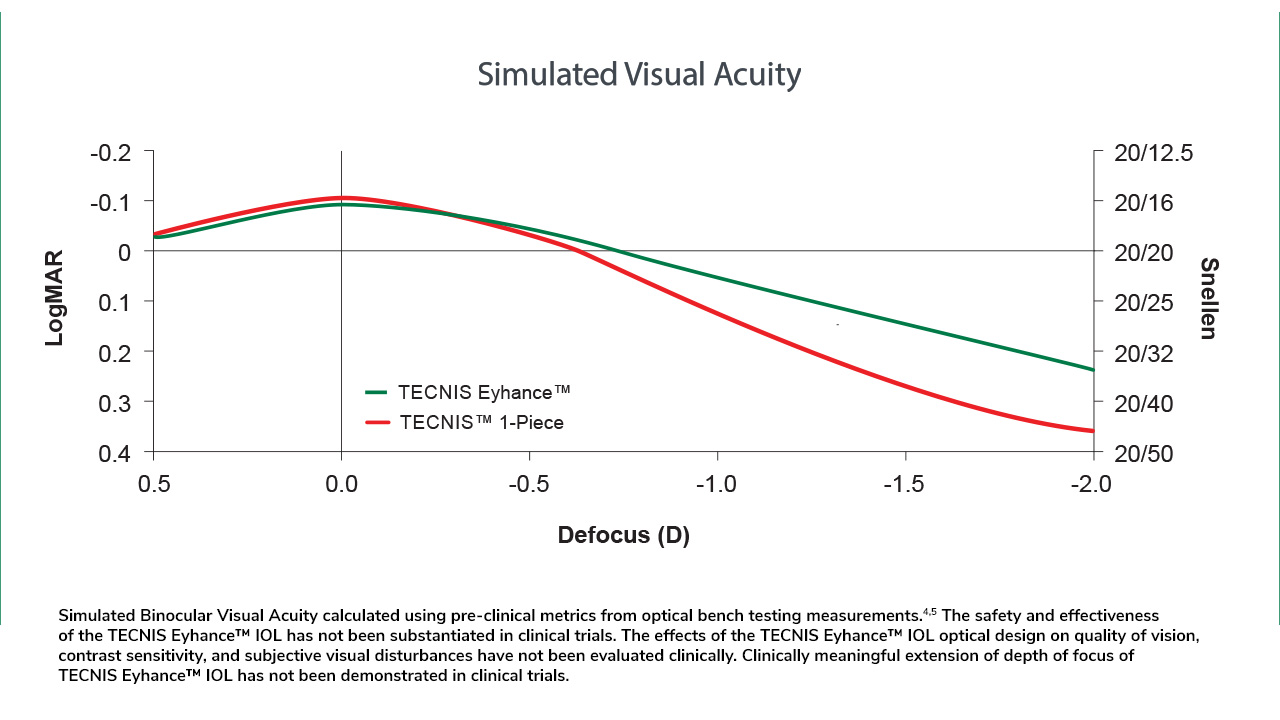
Increased Depth of Focus4
Uncompromised Quality5,6
Best-in-Category† Contrast7
Uncompromised Quality
Continuous increase in power from edge to center is designed to slightly extend depth of focus*5,6 while maintaining correction of spherical aberrations.10
100% of eyes with ≤5° rotation at 3-months post-op‡12
Mean rotation of TECNIS™ Toric II was <1° at 1 week, 1 month, and 3 months.
Learn More
With your consent, we will use your information to send you information about our products and services tailored to your interests through email. You may withdraw your consent at any time.
Please read our Privacy Policy.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Footnotes
* The TECNIS Eyhance™ IOLs are designed to slightly extend the depth of focus compared to the TECNIS™ 1-Piece IOL, Model ZCB00 as measured in bench testing.
** Best contrast and low light performance day and night vs Acrysof® IQ (SN60WF), Clareon® (CNA0T0), Vivinex™ (XY1), enVista® (MX60E) and Acrysof® (SA60AT).
† Best contrast and low-light performance day and night versus Acrysof® IQ (SN60WF), Clareon® (CNA0T0), Vivinex™ (XY1), enVista® (MX60E) and Acrysof® (SA60AT).
¶ Compared to AcrySof® Toric and enVista® Toric IOLs
# Based on a retrospective chart review that looked at the rate of surgical IOL repositioning due to clinically significant IOL rotation comparing case records for 993 eyes implanted with TECNIS™ Toric II (N=308), AcrySofR Toric (N=362), enVistaR Toric IOLs (N=270), or TECNIS™ Toric I (N=53). Outcomes differ from the pivotal investigation data in the product labeling and was collected using different measurement methods, study design and clinical conditions.
‡ Based on data from 200 eyes after 3 months postoperative follow-up in a postmarket prospective, multicenter, single-arm, open-label study in the US. Outcomes different from the pivotal investigation data in the product labeling and was collected using different measurement methods, study design and clinical conditions.
REFERENCES
1. Z311769E TECNIS Eyhance™ IOL with TECNIS SIMPLICITY™ Delivery System US DFU.
2. Z311770E TECNIS Eyhance™ Toric II IOL with TECNIS SIMPLICITY™ Delivery System DFU.
3. Data on File, Johnson & Johnson Surgical Vision, Inc. 2021 DOF2021CT4002.
4. Data on file DOF2021CT4006.
5.Data on file. 2022024DOF4003.
6. Data on file. 2024DOF4005.
7. Data on file. 2023CT4023.
8. Alarcon et al. Biomedical Optics Express 7.5 (2016).
9. Alarcon et al. Journal of Refractive Surgery (2020).
10. Data on file. 2024DOF4015.
11. Hu EH. Repositioning Rates of Toric IOLs Implanted in Cataract Surgery Patients: A Retrospective Chart Review. Clin Ophthalmol. 2023
Dec 23;17:4001-4007. doi: 10.2147/OPTH.S441524. PMID: 38152615; PMCID: PMC10752012.
12. Data on file. DOF2021CT4019.
INDICATIONS and IMPORTANT SAFETY INFORMATION for TECNIS Eyhance™ and TECNIS Eyhance™ Toric II IOLs with TECNIS SIMPLICITY™ Delivery System
Rx Only
INDICATIONS FOR USE
The TECNIS SIMPLICITY™ Delivery System is used to fold and assist in inserting the TECNIS Eyhance™ IOL for the visual correction of aphakia in adult patients in whom a cataractous lens has been removed by extracapsular cataract extraction. The lens is intended to be placed in the capsular bag.
The TECNIS SIMPLICITY™ Delivery System is used to fold and assist in inserting the TECNIS Eyhance™ Toric II IOLs for the visual correction of aphakia and pre-existing corneal astigmatism of one diopter or greater in adult patients with or without presbyopia in whom a cataractous lens has been removed by phacoemulsification and who desire reduction in residual refractive cylinder. The lens is intended to be placed in the capsular bag.
WARNINGS
Physicians considering lens implantation under any of the following circumstances should weigh the potential risk/benefit ratio:
1. Patients with any of the following conditions may not be suitable candidates for an intraocular lens because the lens may exacerbate an existing condition, may interfere with diagnosis or treatment of a condition or may pose an unreasonable risk to the patient’s eyesight. These conditions are not specific to the design of the lens and are attributed to cataract surgery and IOL implantation in general:
a. Patients with recurrent severe anterior or posterior segment inflammation or uveitis of unknown etiology, or any disease producing an inflammatory reaction in the eye.
b. Patients in whom the intraocular lens may affect the ability to observe, diagnose or treat posterior segment diseases.
c. Surgical difficulties at the time of cataract extraction, which may increase the potential for complications (e.g., persistent bleeding, significant iris damage, uncontrolled positive pressure or significant vitreous prolapse or loss).
d. A compromised eye due to previous trauma or developmental defects in which appropriate support of the IOL is not possible.
e. Circumstances that would result in damage to the endothelium during implantation.
f. Suspected microbial infection.
g. Patients in whom neither the posterior capsule nor the zonules are intact enough to provide support for the IOL.
h. Congenital bilateral cataracts.
i. Previous history of, or a predisposition to, retinal detachment.
j. Patients with only one good eye with potentially good vision.
k. Medically uncontrollable glaucoma.
l. Corneal endothelial dystrophy.
m. Proliferative diabetic retinopathy.
n. Children under the age of 2 years are not suitable candidates for intraocular lenses.
2. The lens should be placed entirely in the capsular bag. Do not place the lens in the ciliary sulcus.
3. Johnson & Johnson Surgical Vision, Inc. single-use medical devices are labeled with instructions for use and handling to minimize exposure to conditions which may compromise the product, patient, or the user. When used according to the directions for use, the delivery system minimizes the risk of infection and/or inflammation associated with contamination.
4. The reuse/resterilization/reprocessing of Johnson & Johnson Surgical Vision, Inc. single-use devices may result in physical damage to the medical device, failure of the medical device to perform as intended, and patient illness or injury due to infection, inflammation, and/or illness due to product contamination, transmission of infection, and lack of product sterility.
5. The clinical study for the TECNIS™ Toric 1-Piece IOL did not show evidence of effectiveness for the treatment of preoperative corneal astigmatism of less than one diopter.
6. Rotation of the toric lens from its intended axis can reduce its astigmatic correction. Misalignment greater than 30° may increase postoperative refractive cylinder. If necessary, lens repositioning should occur as early as possible prior to lens encapsulation.
7. Do not attempt to disassemble, modify or alter the delivery system or any of its components, as this can significantly affect the function and/or structural integrity of the design.
8. Do not use if the cartridge of the delivery system is cracked or split prior to implantation.
9. Do not implant the lens if the rod tip does not advance the lens or if it is jammed in the delivery system.
10. During initial lens advancement, quick advancement of the plunger is needed. Do not stop or reverse while advancing the plunger. Doing so may result in improper folding of the lens.
11. After initial lens advancement and the half turn rotation step, do not move the plunger forward until ready for lens implantation. Doing so may result in the lens being stuck in the cartridge.
12. The lens and delivery system should be discarded if the lens has been folded within the cartridge for more than 10 minutes. Not doing so may result in the lens being stuck in the cartridge.
PRECAUTIONS
1. The safety and effectiveness of the TECNIS Eyhance™ IOL and TECNIS Eyhance™ Toric II IOL has not been substantiated in clinical trials. The effects of the TECNIS Eyhance™ IOL optical design on quality of vision, contrast sensitivity, and subjective visual disturbances (glare, halo, etc.) have not been evaluated clinically. MTF testing of the TECNIS Eyhance™ IOL may aid the Surgeon in understanding the theoretical image quality expected with the TECNIS Eyhance™ IOL compared to other JJSV monofocal IOLs (AAB00 and ZCB00). However, these do not fully assess all aspects of clinical difficulties under all conditions. Surgeons must weigh the potential benefits of the modified optical design of the TECNIS Eyhance™ IOL against the potential for risks and the lack of clinical data to characterize the impact of the TECNIS Eyhance™ IOL optical design on contrast sensitivity and subjective visual disturbance. These considerations may be especially relevant to patients with certain pre-existing ocular conditions (prior corneal refractive surgery, irregular corneal astigmatism, severe corneal dystrophy, macular disease, optic nerve atrophy, etc.) or intraoperative conditions (posterior capsular rupture, complications in which the IOL stability could be compromised, inability to place IOL in capsular bag, etc.).
2. Prior to surgery, the surgeon must inform prospective patients of the possible risks and benefits associated with the use of this device and provide a copy of the patient information brochure to the patient.
3. Some autorefractors utilize only the central part of the IOL to calculate the refraction of the eye and that is the region where the TECNIS Eyhance™ deviates from the monofocal design which could result in a wrong estimation of the refraction. Manual refraction with maximum plus technique is strongly recommended.
4. Recent contact lens usage may affect the patient’s refraction; therefore, for patients who wear contact lenses, surgeons should establish corneal stability without contact lenses prior to determining IOL power.
5. The lens is designed for optimum visual performance when emmetropia is targeted.
6. This is a single use device, do not re-sterilize the lens or the delivery system. Most sterilizers are not equipped to sterilize the soft acrylic material and the preloaded inserter material without producing undesirable side effects.
7. Do not store the device in direct sunlight or at a temperature under 5°C (41°F) or over 35°C (95°F).
8. Do not autoclave the delivery system.
9. Do not advance the lens unless ready for lens implantation.
10. The contents are sterile unless the package is opened or damaged.
11. The recommended temperature for implanting the lens is at least 17°C (63°F).
12. The use of balanced salt solution or viscoelastics is required when using the delivery system. For optimal performance when using OVD, use the HEALON™ family of viscoelastics. The use of balanced salt solution with additives has not been studied for this product.
13. Do not use if the delivery system has been dropped or if any part was inadvertently struck while outside the shipping box. The sterility of the delivery system and/or the lens may have been compromised.
14. Do not leave the lens in a folded position more than 10 minutes.
15. When the delivery system is used improperly, the lens may not be delivered properly (i.e., haptics may be broken). Please refer to the specific instructions for use provided.
16. Carefully remove all viscoelastic and do not over-inflate the capsular bag at the end of the case. Residual viscoelastic and/or over-inflation of the capsular bag may allow the lens to rotate, causing misalignment of the TECNIS Eyhance™ Toric II IOL with the intended axis of placement.
17. The use of methods other than the TECNIS™ Toric Calculator to select cylinder power and appropriate axis of implantation were not assessed in the clinical study for the TECNIS™ Toric 1-Piece IOLs. and may not yield similar results. Accurate keratometry and biometry, in addition to the use of the TECNIS™ Toric Calculator (www.TecnisToricCalc.com) are recommended to achieve optimal visual outcomes.
18. The safety and effectiveness of the TECNIS™ Eyhance Toric II IOLs have not been substantiated in patients with the following preexisting ocular conditions and intraoperative complications (see below). Careful preoperative evaluation and sound clinical judgment should be used by the surgeon to decide the benefit/risk ratio before implanting a lens in a patient with one or more of these conditions.
Before Surgery
- Choroidal hemorrhage
- Chronic severe uveitis
- Concomitant severe eye disease
- Extremely shallow anterior chamber
- Medically uncontrolled glaucoma
- Microphthalmos
- Non-age-related cataract
- Proliferative diabetic retinopathy (severe)
- Severe corneal dystrophy
- Severe optic nerve atrophy
- Irregular corneal astigmatism
During Surgery
- Excessive vitreous loss
- Capsulotomy by any technique other than a circular tear
- The presence of radial tears known or suspected at the time of surgery
- Situations in which the integrity of the circular tear cannot be confirmed by direct visualization
- Cataract extraction by techniques other than phacoemulsification or liquefaction
- Situations where the need for a large capsulotomy can be anticipated (e.g., diabetics, retinal detachment in the fellow eye, peripheral retinal pathology, etc.)
- Capsular rupture
- Significant anterior chamber hyphema
- Uncontrollable positive intraocular pressure
- Zonular damage
19. The PCA is based on an algorithm that combines published literature (Koch et.al, 2012) and a retrospective analysis of data from a TECNIS™ Toric multi-center clinical study. The PCA algorithm for the selection of appropriate cylinder power and axis of implantation was not assessed in a prospective clinical study and may yield results different from those in the TECNIS™ Toric intraocular lens labeling. Please refer to the Johnson & Johnson Surgical Vision, Inc. Toric Calculator user manual for more information.
20. All preoperative surgical parameters are important when choosing a toric lens for implantation, including preoperative keratometric cylinder (magnitude and axis), incision location, surgeon’s estimated surgically induced astigmatism (SIA) and biometry. Variability in any of the preoperative measurements can influence patient outcomes, and the effectiveness of treating eyes with lower amounts of preoperative corneal astigmatism.
21. All corneal incisions were placed temporally in the clinical study for the TECNIS™ Toric 1-Piece IOLs. If the surgeon chooses to place the incision at a different location, outcomes may be different from those obtained in the clinical study. Note that the TECNIS™ Toric Calculator incorporates the surgeon’s estimated SIA and incision location when providing IOL options.
ADVERSE EVENTS
Potential adverse events during or following cataract surgery with implantation of an IOL may include but are not limited to: endophthalmitis/intraocular infection, hypopyon, hyphema, IOL dislocation, cystoid macular edema, pupillary block, retinal detachment/tear, persistent corneal stromal edema, persistent iritis, persistent raised IOP (intraocular pressure) requiring treatment, acute corneal decompensation, secondary surgical intervention (including implant repositioning, removal, or other surgical procedure) and any other adverse event that leads to permanent visual impairment or requires surgical or medical intervention to prevent permanent visual impairment. The most frequently reported cumulative adverse event that occurred during the SENSAR™ 1-Piece IOL clinical trial was cystoid macular edema which occurred at a rate of 3.3%. Other reported events included secondary surgical intervention (pars plana vitrectomy with membrane peel) which occurred at a rate of 0.8% and lens exchange (torn haptic related to improper loading technique) which occurred at a rate of 0.8%. The most frequently reported cumulative adverse event that occurred during the TECNIS™ Toric 1-Piece IOL clinical trial was surgical re-intervention which occurred at a rate of 3.4% (lens repositioning procedures and retinal repair procedures). Other reported events included cystoid macular edema which occurred at a rate of 2.9% and retinal detachment which occurred at a rate of 0.6%.
ATTENTION: Reference the Directions for Use for a complete listing of Indications and Important Safety Information.
2024PP19946