*The lenses are indicated for reducing refractive error up to 6.00D of myopia and up to 1.50D of astigmatism. Results may vary by patient and prescription level.
†Previously marketed under Menicon Z Night. Initial CE approval for Orthokeratology was granted in 2006.
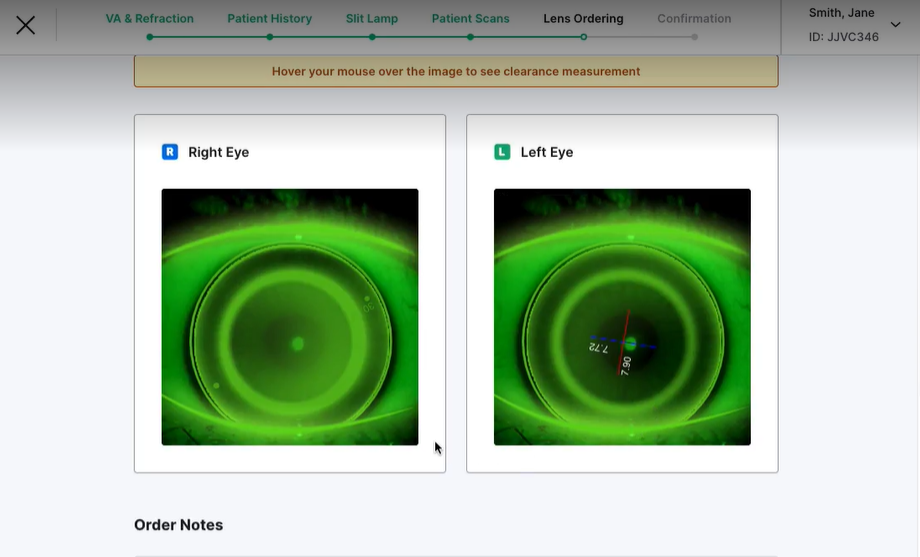
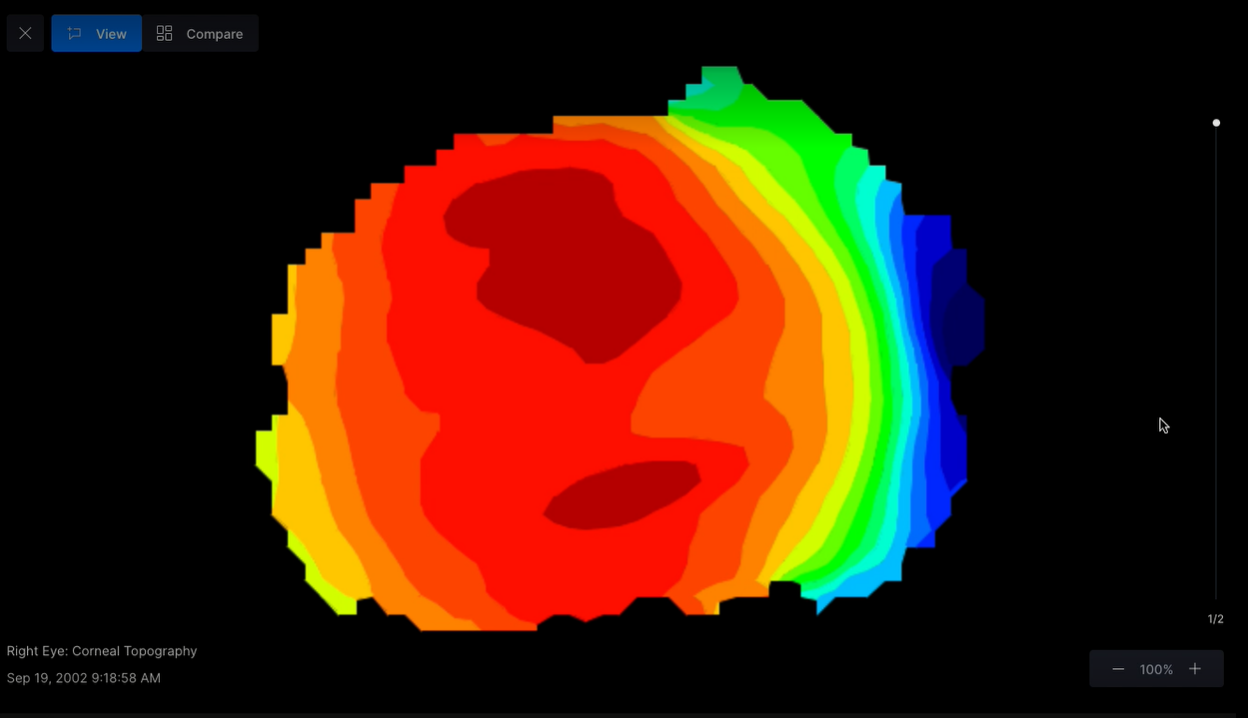
‡Final lens fit is determined by the ECP – software is for decision support only
^The lens is approved for correcting astigmatism of up to 1.50D
References
1. JJV Data on File 2023. ACUVUE® Abiliti™ Overnight Therapeutic Lenses for Myopia Management - Consolidated, Approved Claims List; U.S. Only Claims
2. Data on File 2023. Instruction For Use (FDA)
3. Data on File 2023. Menicon Initial CE approval 2006
4. Data on File 2023. Menicon Design History file
6. Lions Club International Foundation. Real impact: By the numbers. Available from: https://www.lionsclubs.org/en/resources-for-members/resource-center/sight-for-kids
Important safety information:
ACUVUE® Abiliti™ Overnight Therapeutic (tisilfocon A) Contact Lenses are indicated for use in the management of myopia. They are indicated for overnight wear for the temporary reduction of myopia and should only be disinfected using a chemical disinfection system. As with any contact lens, eye problems, including corneal ulcers, can develop. Some wearers may experience mild irritation, itching or discomfort. These lenses should not be prescribed if patients have any eye infection, or experience eye discomfort, excessive tearing, vision changes, redness, other eye problems, or if patients have any allergy to any ingredient in a solution which is to be used to care for these lenses. Complete information is also available from Johnson & Johnson Vision Care, Inc. by calling 1-877-334-3937, or by visiting www.seeyourabiliti.com.
PP2023ABL4104